Unit 5- Morden Physics
Chapter 25 – Solids
Introduction
Matter exists in nature in three states, namely solid, liquid and gas. Depending upon the nature of bond between atoms, ions or molecules, matter divided into three categories.
The solids are characterized by the definite shape as well as definite volume.Depending upon the nature and arrangement of atoms, ions or molecules, solids are classified into two categories.
i) Crystalline solid
ii) Amorphous solid
i) Crystalline solid:-
A solid in which the constituents atoms, ions or molecules are arranged in definite pattern in three dimension is called crystalline solid. For instance, Nacl, diamond, graphite
Chacteristic of crystalline solids.
Chacteristic of crystalline solids
- They have regular and periodic arrangement of atoms or ions or
molecules. - They have long range spatial order in their structure.
- Their composition is homogenous
- They have high menting points
- They do not have the same physical properties in all direction.

ii) Amorphous solid:-
A solid in which the constituents atoms,ions or molecules are arranged in random manner is called amorphous. For example glass, wood.
Characteristics of amorphous solids
- They have not regular and periodic arrangement of atoms or ions
or molecules. - They have short range spatial ordere in their structure.
- They have the same physical properties in all direction.
- Their compositions may not be homogenous.
- They do not have sharp menting point.

Energy levels:
Energy level is a quantized energy value of an atom. An atom contains electrons that are in continuous movement around the atomic nucleus. These electrons have discrete energy values; thus, we say the energy is quantized. We call each quantized energy value an
energy level.
Energy bands:
Energy band is a continuous combination of energy levels of electrons in a molecule. Therefore this term is used mainly in molecular levelexplanations. When there are multiple atoms combined with each other to form a molecule, the electrons get placed in molecularorbitals.
A molecular orbital is the type of orbital that is formed by the combination of two atomic orbitals
The band theory of solids is different from the others because the atoms are arranged very close to each other such that the energy levels of the outermost orbital electrons are affected. But the energy level of the innermost electrons is not affected by the neighboring.
Atoms:
In-band theory of solid, there are many energy bands but the following are the three most important energy bands in solids:
- Valence band
- Conduction band
- Forbidden band
Valence band:
The energy band that consists of valence electrons energy levels, is known as the valence band. The valence band is present below the conduction band and the electrons of this band are loosely bound to the nucleus of the atom.
Conduction band:
The energy band that consists of free electrons energy levels, is known as the conduction band. For electrons to be free, external energy must be applied such that the valence electrons get pushed to the conduction band and become free.
Forbidden band:
The energy gap between the valence band and the conduction band is known as the forbidden band which is also known as the forbidden gap. The electrical conductivity of a solid is determined from the forbidden gap and also the classification of the materials as conductors, semiconductors, and insulators.
What is the difference Between Metals, Insulators, and Semiconductors on the basis of their energy bands?
Metals:
In metals, the conduction band is either partially filled or the valence band is partially empty. There are electrons that behave as free electrons as they shift to higher energy levels by acquiring energy above the Fermi level in the conduction band. There is no forbidden energy gap in the metals. Since there is no forbidden gap, the number of electrons available for the conduction is more increasing the conductivity of the material. Metals behave as a conductor
because of the movement of the free electrons when a small amount of electric current is applied.
Insulators:
In insulators, the valence band is completely filled while the conduction band is empty. This results in a large energy gap. Since the energy gap between the conduction band and the valence band is more, there is no movement of electrons from the valence band to the conduction band.
Semiconductor:
In a semiconductor, the valence band is completely filled with electrons while the conduction band is empty. The energy gap between the bands is less. For electrons to jump from the
valence band to the conduction band, room temperature needs to be maintained. If the temperature is 0K, there is no transfer of electrons from the valence band to the conduction band.

Semi-Conductor
Semiconductors are the materials which have a conductivity between conductors (generally metals) and non-conductors or insulators (suchas ceramics). Semiconductors can be compounds such as gallium arsenide or pure elements, such as germanium or silicon. Physics
explains the theories, properties and mathematical approach governing semiconductors.
Examples of Semiconductors:
Gallium arsenide, germanium, and silicon are some of the most commonly used semiconductors. Silicon is used in electronic circuit fabrication and gallium arsenide is used in solar cells, laser diodes, etc.
Classicification of semiconductor

Intrinsic semiconductor
An intrinsic type of semiconductor material is made to be very pure chemically. It is made up of only a single type of element

Germanium (Ge) and Silicon (Si) are the most common type of intrinsic semiconductor elements. They have four valence electrons (tetravalent). They are bound to the atom by covalent bond at absolute zero temperature.
When the temperature rises, due to collisions, few electrons are unbounded and become free to move through the lattice, thus creating an absence in its original position (hole). These free
electrons and holes contribute to the conduction of electricity in the semiconductor. The negative and positive charge carriers are equal in number.
The thermal energy is capable of ionizing a few atoms in the lattice, and hence their conductivity is less.
Extrinsic semiconductor
The conductivity of semiconductors can be greatly improved by introducing a small number of suitable replacement atoms called IMPURITIES. The process of adding impurity atoms to the pure semiconductor is called DOPING. An extrinsic semiconductor can be further classified into:
- N-type Semiconductor
- P-type Semiconductor
p-type semiconductor
When trivalent impurities like Boron (B) , Aluminum (Al), Gallium (Ga), Indium (In) etc is added to pure semiconductor like germanium or silicon the conductivity of the crystal increase due to the excess of hole and such type of semiconductor is called p- type semiconductor. Where the impurities atoms are called acceptors.
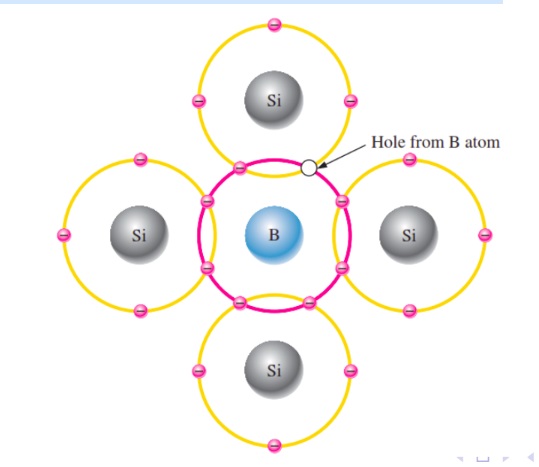
With the increase in the number of impurities, holes (the positive charge carriers) are increased. Hence, it is called p-type semiconductor.
Crystal as a whole is neutral, but the acceptors become an immobile negative ion. As conduction is due to a large number of holes, the holes in the p-type semiconductor are MAJORITY CARRIERS and electrons are MINORITY CARRIERS.
N-type semiconductor
When pentavalent impurities like Arsenic (As) , phosphorous (P), antimony (Sb) etc is added to pure semiconductor like germanium or silicon the conductivity of the crystal increase due to the increase ofelectron and such type of semiconductor is called N- type semiconductor. Where the impurities atoms are called donors.

Since the number of free electron increases by the addition of an impurity, the negative charge carriers increase. Hence, it is called n-type semiconductor.
Crystal as a whole is neutral, but the donor atom becomes an immobile positive ion. As conduction is due to a large number of free electrons, the electrons in the n-type semiconductor are the MAJORITY CARRIERS and holes are the MINORITY CARRIERS.






























